Frequently Asked Questions
Explore frequently asked questions about ENJAYMO and Cold Agglutinin Disease (CAD) here
CAD is a rare, complement-mediated, autoimmune hemolytic anemia with potentially serious consequences.1-3 In patients with CAD, cold agglutinins (IgM autoantibodies against RBC antigens) can cause clinical symptoms related to RBC agglutination in cooler parts of the body and ultimately result in hemolytic anemia. Hemolysis in CAD is driven by C1 activation of the classical complement pathway, when cold agglutinins recruit and activate C1, typically at body temperatures under 98 °F (37 °C).4,5
References: 1. Berentsen S, Beiske K, Tjønnfjord GE. Primary chronic cold agglutinin disease: an update on pathogenesis, clinical features and therapy. Hematology. 2007;12(5):361-370. doi:10.1080/10245330701445392 2. Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839-848. doi:10.1182/bloodadvances.2017004390 3. Broome CM, Cunningham JM, Mullins M, et al. Increased risk of thrombotic events in cold agglutinin disease: a 10-year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628-635. doi:10.1002/rth2.12333 4. Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479-492. doi:10.1016/j.semnephrol.2013.08.001 5. Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141-149. doi:10.1053/j.seminhematol.2018.04.002
The most common symptoms of CAD are hemolytic anemia, profound fatigue, and circulatory symptoms.1-3
Learn more about symptoms and diagnosing CADReferences: 1. Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839-848. doi:10.1182/bloodadvances.2017004390 2. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41(100648):100648. doi:10.1016/j.blre.2019.100648 3. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114-1121. doi:10.1182/blood-2013-02-474437
Chronic hemolysis in CAD leaves patients in constant risk for acute consequences and chronic risks.1-4 Hemolysis in CAD is caused by classical complement pathway–mediated destruction of red blood cells.5 Persistent activation of the classical complement pathway can lead to severe anemia due to the destruction of red blood cells.6-9
View the mechanism of CADReferences: 1. Berentsen S, Beiske K, Tjønnfjord GE. Primary chronic cold agglutinin disease: an update on pathogenesis, clinical features and therapy. Hematology. 2007;12(5):361-370. doi:10.1080/10245330701445392 2. Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839-848. doi:10.1182/bloodadvances.2017004390 3. Broome CM, Cunningham JM, Mullins M, et al. Increased risk of thrombotic events in cold agglutinin disease: a 10-year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628-635. doi:10.1002/rth2.12333 4. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114-1121. doi:10.1182/blood-2013-02-474437 5. Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141-149. doi:10.1053/j.seminhematol.2018.04.002 6. Berentsen S. New insights in the pathogenesis and therapy of cold agglutinin-mediated autoimmune hemolytic anemia. Front Immunol. 2020;590(11):1-13. doi:10.3389/fimmu.2020.00590 7. Landsem A, Nielsen EW, Fure H, et al. C1-inhibitor efficiently inhibits Escherichia coli-induced tissue factor mRNA up-regulation, monocyte tissue factor expression and coagulation activation in human whole blood. Clin Exp Immunol. 2013;173(2):217-229. doi:10.1111/cei.12098 8. Cofiell R, Kukreja A, Bedard K, et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125(21):3253-3262. doi:10.1182/blood-2014-09-600411 9. Weitz IC, Razavi P, Rochanda L, et al. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res. 2012;130(3):361-368. doi:10.1016/j.thromres.2012.04.001
CAD is a well-characterized disease that is defined by classical complement-dependent chronic hemolysis and a clonal, low-grade B-cell lymphoproliferative disorder without an underlying condition. By contrast, CAS is a transient condition, secondary to bacterial or viral infections (ie, Mycoplasma pneumoniae or Epstein-Barr virus), overt malignancies, and autoimmune disorders. Additionally, CAS is usually self-remitting as the associated condition resolves. In contrast, CAD is a chronic condition.1-3
References: 1. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41(100648):100648. doi:10.1016/j.blre.2019.100648 2. Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):226-231. doi:10.1182/asheducation-2016.1.226 3. Berentsen S, Tjønnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26(3):107-115. doi:10.1016/j.blre.2012.01.002
CADENZA was a phase 3, placebo-controlled, global, multicenter, randomized, double-blind trial to demonstrate the efficacy and safety of ENJAYMO in 42 patients with CAD and no transfusion history.1,2*
Composite primary endpoint: Efficacy was based on the proportion of patients who achieved an Hb level increase of ≥1.5 g/dL from baseline at the treatment assessment time point (TAT) (mean value from Weeks 23, 25, and 26) and both transfusion avoidance and no additional treatment beyond what was permitted per protocol in the trial from Week 5 through Week 26.1,2
Secondary endpoints assessed: Effect on Hb (mean change from baseline at TAT), improvements in the symptoms and impacts of fatigue (mean change from baseline at TAT in FACIT-Fatigue score), and laboratory measures of hemolysis (mean change from baseline at TAT in bilirubin and LDH).1,2
Long-term safety and durability: Following completion of the 6-month treatment period, patients continued to receive ENJAYMO in a long-term safety and durability of response extension phase for an additional 12 months.1,2
*Patients with confirmed CAD and no history of transfusion within 6 months or >1 in 12 months prior to enrollment (N=42). Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease (bone marrow involvement <10%) were not excluded.
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Berentsen S, Barcellini W, et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood. 2022;140(9):980-991. doi.org/10.1182/blood.2021014955
The CADENZA study population consisted of patients with a confirmed diagnosis of CAD based on chronic hemolysis, polyspecific direct antiglobulin test (DAT), monospecific DAT specific for C3d, cold agglutinin titer ≥64 at 4 °C, and IgG DAT ≤1+ and no history of transfusion within 6 months or more than 1 blood transfusion in the 12 months prior to enrollment.1
Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease (bone marrow involvement <10%) were not excluded.1
Reference: 1. ENJAYMO. Prescribing information.
-
The primary endpoint was a composite of Hb improvement, avoidance of transfusions, and avoidance of protocol-prohibited CAD medication1,2
- Hemoglobin improvement defined as an increase in baseline Hb level ≥1.5 g/dL at the treatment assessment time point (TAT)*
- Transfusion avoidance from Week 5 through Week 26
- No treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26
- The secondary endpoints assessed the effect on Hb and laboratory measures of hemolysis (mean change from baseline in bilirubin and LDH) at the TAT.* Symptoms and impacts of fatigue were assessed by mean change from baseline in FACIT-Fatigue score.1,2
*Defined as the mean value from Weeks 23, 25, and 26.
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Berentsen S, Barcellini W, et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood. 2022;140(9):980-991. doi.org/10.1182/blood.2021014955
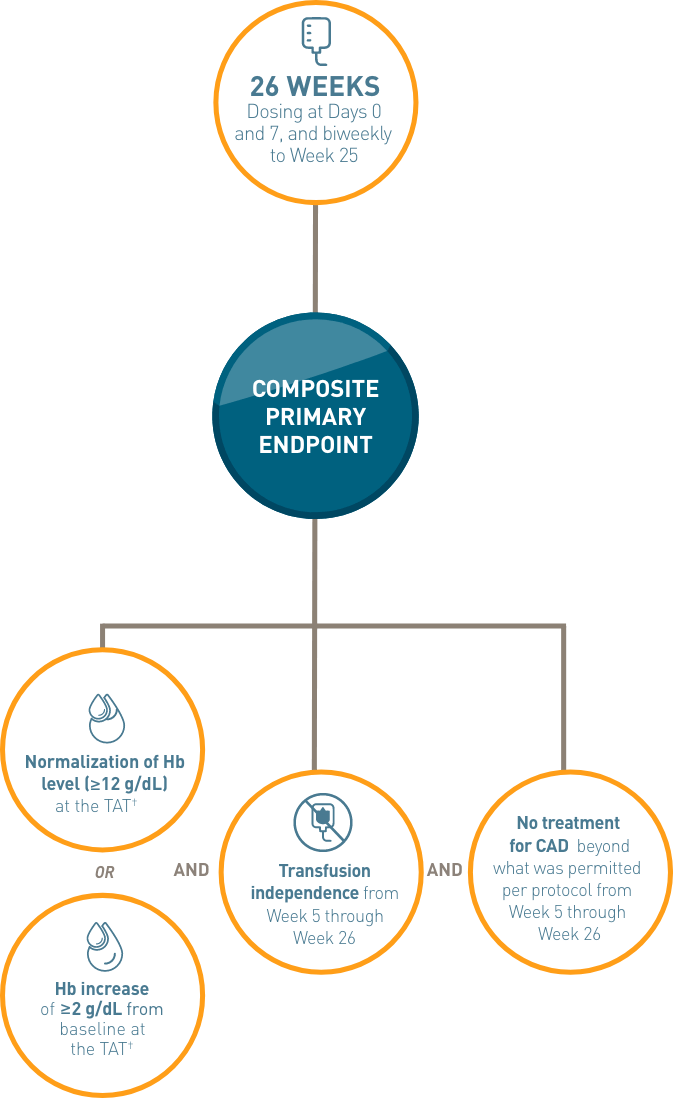
CARDINAL was a phase 3, global, multicenter, open-label, single-arm, 6-month trial to demonstrate the efficacy and safety of ENJAYMO in 24 patients with CAD who received ≥1 transfusion during the preceding 6 months.1,2*
Composite primary endpoint: Efficacy was based on the proportion of patients who achieved an Hb level ≥12 g/dL or an increase of ≥2 g/dL from baseline at the treatment assessment time point (TAT) (mean value from Weeks 23, 25, and 26) and both transfusion avoidance and no additional treatment beyond what was permitted per protocol in the trial from Week 5 through Week 26.1,2
Secondary endpoints assessed: Effect on Hb and laboratory measures of hemolysis (mean change from baseline in bilirubin and LDH) at the TAT.2†
Long-term safety and durability: Following completion of the 6-month treatment period, patients continued to receive ENJAYMO in a long-term safety and durability of response extension phase for an additional 24 months.1,2

Secondary endpoints assessed: Effect on Hb and laboratory measures of hemolysis (mean change from baseline in bilirubin and LDH) at the TAT.†
*Patients with confirmed CAD and a recent blood transfusion in 6 months prior to enrollment received ENJAYMO (N=24). Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease were not excluded.
†Defined as the mean value from Weeks 23, 25, and 26.
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Barcellini W, D’Sa S, et al. Sutimlimab in Cold Agglutinin Disease. N Engl J Med. 2021;384(14):1323-1334. doi:10.1056/NEJMoa2027760
The CARDINAL study population consisted of patients with a confirmed diagnosis of CAD based on chronic hemolysis, polyspecific direct antiglobulin test (DAT), monospecific DAT specific for C3d, cold agglutinin titer ≥64 at 4 °C, and IgG DAT ≤1+ and a recent blood transfusion in the 6 months prior to enrollment.1
Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease were not excluded. Patients with low-grade lymphoproliferative disease were categorized as patients with bone marrow involvement <10%.1,2
References: 1. ENJAYMO. Prescribing information. 2. Data on file. Recordati Rare Diseases.
No, the label does not restrict or limit the use of ENJAYMO to patients with a history of transfusion. ENJAYMO has been studied in patients with and without a history of transfusion. ENJAYMO is indicated for the treatment of hemolysis in adults with CAD.1
Reference: 1. ENJAYMO. Prescribing information.
-
The primary endpoint was a composite of Hb improvement, avoidance of transfusions, and avoidance of protocol-prohibited CAD medication1,2
- Hemoglobin improvement defined as Hb level ≥12 g/dL at the TAT point OR Hb increase of ≥2 g/dL from baseline at the TAT*
- Transfusion avoidance from Week 5 through Week 26
- No treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26
- The secondary endpoints assessed the effect on Hb and laboratory measures of hemolysis (mean change from baseline in bilirubin and LDH) at the TAT1,2*
*Defined as the mean value from Weeks 23, 25, and 26.
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Barcellini W, D’Sa S, et al. Sutimlimab in Cold Agglutinin Disease. N Engl J Med. 2021;384(14):1323-1334. doi:10.1056/NEJMoa2027760
In the CADENZA trial,* 73% (16/22) of patients met the composite endpoint,† which included achieving an Hb increase1,2:
ENJAYMO provided rapid and sustained improvement in Hb levels through Week 261:
- Achieved 2.02 g/dL mean increase at Week 3
- >2 g/dL mean increase was maintained at all time points starting at Week 3
Hb data in the CARDINAL trial‖ demonstrated improvement consistent with the CADENZA trial. In the CARDINAL trial, 54% (13/24) of patients met the composite endpoint, which included achieving an Hb increase1,3:
ENJAYMO provided a rapid and sustained improvement in Hb levels through Week 261:
- Achieved 2.29 g/dL mean increase in Hb levels at Week 3 (SE: 0.308)
- Achieved 3.18 g/dL mean increase in Hb at TAT (SE: 0.476)
*CADENZA, a phase 3, placebo-controlled, global, multicenter, randomized, double-blind trial, demonstrated efficacy and safety of ENJAYMO in patients with CAD and no history of transfusion. Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease (bone marrow involvement <10%) were not excluded.1,2
†Two patients discontinued prior to Week 23, and their status was considered unknown for the purposes of this analysis.2
‡Defined as the mean value from Weeks 23, 25, and 26.1
§Prohibited therapies included rituximab alone or in combination with cytotoxic agents.1
‖CARDINAL, a phase 3, open-label, single-arm, 6-month trial, demonstrated efficacy and safety of ENJAYMO in patients with CAD who received ≥1 transfusion during the preceding 6 months. Patients with CAS secondary to infection, rheumatologic disease, SLE, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease were not excluded.1,3
¶From baseline at TAT. Mean Hb at baseline: 8.6 g/dL. Hb level ≥12 g/dL achieved in 38% of patients (9/24); increase in Hb level of ≥2 g/dL achieved in 63% of patients (15/24).1
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Berentsen S, Barcellini W, et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood. 2022;140(9):980-991. doi.org/10.1182/blood.2021014955 3. Röth A, Barcellini W, D’Sa S, et al. Sutimlimab in Cold Agglutinin Disease. N Engl J Med. 2021;384(14):1323-1334. doi:10.1056/NEJMoa2027760
Bilirubin is a key endpoint for CAD because the hemolysis is predominantly extravascular in CAD and takes place in the liver. LDH is mainly a marker of intravascular hemolysis, which is less common in CAD.1-3
References: 1. Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141-149. doi:10.1053/j.seminhematol.2018.04.002 2. Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41(100648):100648. doi:10.1016/j.blre.2019.100648 3. Bilirubin test. Mayo Clinic. October 8, 2022. Accessed November 8, 2022. https://www.mayoclinic.org/tests-procedures/bilirubin/about/pac-20393041
ENJAYMO, a proximal classical complement C1s inhibitor, increases susceptibility to serious infections, including infections caused by encapsulated bacteria such as Neisseria meningitidis (any serogroup, including non-groupable strains), Streptococcus pneumoniae, and Haemophilus influenzae type B.1
Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. Vaccination does not eliminate the risk of serious encapsulated bacterial infections.
Advise patients of the risk of serious infection. Inform patients that they are required to receive vaccinations against these bacteria according to current medical guidelines prior to initiation of and during treatment with ENJAYMO. Educate patients on the symptoms of infections and advise them to seek immediate medical attention if any new symptoms of infection occur.1
There were no meningococcal infections with ENJAYMO seen in the clinical trials.2-4
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Berentsen S, Barcellini W, et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood. 2022;140(9):980-991. doi.org/10.1182/blood.2021014955 3. Röth A, Barcellini W, D’Sa S, et al. Sutimlimab in Cold Agglutinin Disease. N Engl J Med. 2021;384(14):1323-1334. doi:10.1056/NEJMoa2027760 4. Data on file. Recordati Rare Diseases.
Based on its mechanism of action, ENJAYMO may potentially increase the risk for developing autoimmune diseases such as SLE. Development of SLE has been associated with inherited classical complement deficiency. Patients with SLE or autoimmune disease with positive antinuclear antibody were excluded from clinical trials with ENJAYMO. Monitor patients being treated with ENJAYMO for signs and symptoms and manage medically.1
There were no cases of SLE reported with ENJAYMO in clinical trials.2,3
References: 1. ENJAYMO. Prescribing information. 2. Röth A, Berentsen S, Barcellini W, et al. Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial. Blood. 2022;140(9):980-991. doi.org/10.1182/blood.2021014955 3. Röth A, Barcellini W, D’Sa S, et al. Sutimlimab in Cold Agglutinin Disease. N Engl J Med. 2021;384(14):1323-1334. doi:10.1056/NEJMoa2027760
Complete or update vaccination against encapsulated bacteria, including Streptococcus pneumoniae and Neisseria meningitidis (serogroups A, C, W, Y and B), according to current ACIP recommendations for patients receiving complement inhibitors at least 2 weeks prior to initiation of ENJAYMO.
If urgent ENJAYMO therapy is indicated in a patient who is not up to date with vaccines for Streptococcus pneumoniae and Neisseria meningitidis administer these vaccines as soon as possible.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections.
Reference: 1. ENJAYMO. Prescribing information.
In CADENZA, serious adverse reactions occurred in 2/22 (9%) patients who received ENJAYMO. Serious adverse reactions included Raynaud's phenomenon (n=1) and febrile infection (n=1).1
Permanent discontinuation of ENJAYMO due to an adverse reaction occurred in 2/22 (9%) patients. These were Raynaud's phenomenon (n=1), acrocyanosis (n=1), and infusion-related reactions (n=1).1
In CARDINAL, serious adverse reactions occurred in 10/24 (42%) patients who received ENJAYMO.1
Permanent discontinuation of ENJAYMO due to an adverse reaction occurred in 2/24 (8%) patients. These were pneumonia klebsiella (n=1) and acrocyanosis (n=2).1
Reference: 1. ENJAYMO. Prescribing information.
The most common adverse reactions in the CADENZA trial (Part A) (incidence ≥18%) were rhinitis, headache, hypertension, acrocyanosis, and Raynaud's phenomenon. The most common adverse reactions in the CARDINAL trial (incidence ≥25%) were urinary tract infection, respiratory tract infection, bacterial infection, dizziness, fatigue, peripheral edema, arthralgia, cough, hypertension, and nausea.1
Reference: 1. ENJAYMO. Prescribing information.
No. A REMS program is not required for ENJAYMO.
Patients can receive an infusion 3 ways: in office, at an infusion center, or at home.*
What to know before, during, and after an infusion1
BEFORE INITIATION
Complete or update vaccination against encapsulated bacteria, including Streptococcus pneumoniae and Neisseria meningitidis (serogroups A, C, W, Y and B), according to current ACIP recommendations for patients receiving complement inhibitors at least 2 weeks prior to initiation of ENJAYMO.
If urgent ENJAYMO therapy is indicated in a patient who is not up to date with vaccines for Streptococcus pneumoniae and Neisseria meningitidis administer these vaccines as soon as possible.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections.
DURING INFUSION
Monitor for infusion-related reactions like shortness of breath, rapid heartbeat, nausea, flushing, headache, hypotension, chest discomfort, pruritus, rash, injection-site reaction, and dizziness.
Slow or stop the infusion if an infusion reaction occurs and institute appropriate supportive measures if signs of hypersensitivity occur.
AFTER INFUSION
Following initial infusion, monitor for 2 hours for signs or symptoms of an infusion and/or hypersensitivity reaction.
For subsequent ENJAYMO infusions, monitor for 1 hour for signs of an infusion reaction.
*Subject to coverage requirements and physician determination.
Reference: 1. ENJAYMO. Prescribing information.
Slow or stop the infusion in case of infusion reaction during ENJAYMO administration. Discontinue ENJAYMO infusion and institute appropriate supportive measures if signs of hypersensitivity reactions, such as cardiovascular instability or respiratory compromise, occur.1
Patients should be monitored for infusion and/or hypersensitivity reactions following completion of infusion with ENJAYMO1:
- At least 2 hours following completion of the initial infusion
- One hour following completion of subsequent infusions
Reference: 1. ENJAYMO. Prescribing information.
If a dose is missed, administer as soon as possible; thereafter, resume dosing every 2 weeks. If the duration after the last dose exceeds 17 days, administer ENJAYMO weekly for 2 weeks, with administration every 2 weeks thereafter.1
Staying on therapy matters—stopping ENJAYMO resulted in recurrent hemolysis and anemia. After the last dose of ENJAYMO in the CADENZA trial, signs of symptoms of recurrent hemolysis were observed. At 9 weeks after the last dose, mean hemoglobin decreased by 2.41 g/dL (SE: 0.373) and mean bilirubin increased by 1.27 mg/dL from last available values during treatment.1
Monitor patients for signs and symptoms of hemolysis if treatment with ENJAYMO is interrupted.1
Reference: 1. ENJAYMO. Prescribing information.
ENJAYMO Patient Solutions is here to support your patients from the start and throughout treatment. Patient Support Services may be able to provide your patients with education services, reimbursement services, and related materials.
Download the ENJAYMO Patient Enrollment FormENJAYMO Financial Assistance Programs may be able to help with the cost of treatment. Access to ENJAYMO at no cost may be available to eligible patients who are uninsured or underinsured. Co-pay assistance may be available for out-of-pocket co-pay or co-insurance costs related to ENJAYMO prescription or infusion costs for eligible patients. Additional terms and conditions apply.*
The ENJAYMO Patient Solutions Co-Pay Program (the “Program”) is not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DoD, TRICARE, or similar federal or state programs including any state pharmaceutical assistance programs. The Program is not valid where prohibited by law and savings may vary depending on patients’ out-of-pocket costs. Recordati Rare Diseases reserves the right to modify or terminate the Program at any time without notice. Patients will receive all Program details upon registration.
Please contact ENJAYMO Patient Solutions at 1-833-223-2428, Monday through Friday, 8 am to 8 pm ET, for more information.
*The ENJAYMO Patient Solutions Co-Pay program (the “Program”) is not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DoD, TRICARE, or similar federal or state programs including any state pharmaceutical assistance programs. The Program is not valid where prohibited by law and savings may vary depending on patients’ out-of-pocket costs. Recordati Rare Diseases reserves the right to modify or terminate the Program at any time without notice. Patients will receive all Program details upon registration.
TRICARE is a registered trademark of the Department of Defense, Defense Health Agency. All rights reserved.
Your patients may call ENJAYMO Patient Solutions at 1-833-223-2428, Monday through Friday, 8 am to 8 pm ET, for assistance.
Yes, and you can access the codes here or download the ENJAYMO Billing and Coding Guide for Reimbursement for information regarding coverage and other reimbursement considerations for ENJAYMO.
-
STEP 1: Enroll your patients in ENJAYMO Patient Solutions
-
STEP 2: Complete vaccination against encapsulated bacteria
-
STEP 3: Determine your patients' ENJAYMO infusion plan
-
STEP 4: Acquire ENJAYMO for your patients*
*Unless a specific specialty distributor is mandated by the patient’s payer, ENJAYMO can be filled at any authorized specialty distributor.
Recordati Rare Diseases is committed to helping ensure all patients with CAD have support and access to treatment with ENJAYMO.
- Case Managers and Therapeutic Education Managers are available to support ENJAYMO patients: Case managers are experienced in helping individuals get started on treatment and assisting with insurance-related needs. Patients enrolled in ENJAYMO Patient Solutions will be assigned a case manager to serve as their single point of contact to help them through the onboarding process for ENJAYMO. Therapeutic Education Managers, or TEMs, are available to provide education about ENJAYMO and CAD. TEMs help raise awareness about CAD in the community and educate patients and caregivers. They engage with patients to provide information and facilitate educational events about ENJAYMO and CAD—they are paid to provide educational services on behalf of Recordati Rare Diseases but do not provide medical advice. TEMs will advise patients to talk to their doctor about any healthcare needs
- ENJAYMO Patient Solutions is designed to increase accessibility to treatment while reducing barriers to starting and staying on therapy
- ENJAYMO Co-Pay Assistance Program may be able to help with eligible patients’ treatment costs, such as out-of-pocket, co-payments or co-insurance, and cost of infusion if they meet the program requirements*
- ENJAYMO Patient Assistance Program provides financial support for eligible patients who are uninsured or underinsured
*The ENJAYMO Patient Solutions Co-Pay program (the “Program”) is not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DoD, TRICARE, or similar federal or state programs including any state pharmaceutical assistance programs. The Program is not valid where prohibited by law and savings may vary depending on patients’ out-of-pocket costs. Recordati Rare Diseases reserves the right to modify or terminate the Program at any time without notice. Patients will receive all Program details upon registration.
TRICARE is a registered trademark of the Department of Defense, Defense Health Agency. All rights reserved.
ENJAYMO is available through the following authorized specialty distributors:
At-home infusion is available through CVS Pharmacy. Contact ENJAYMO Patient Solutions at 1-833-223-2428 for more information and to review eligibility requirements.
ENJAYMO, a C1s inhibitor, blocks C1 upstream in the classical complement pathway. ENJAYMO prevents downstream extravascular hemolysis, the predominant form of hemolysis in CAD, as well as preventing downstream intravascular hemolysis. ENJAYMO does not inhibit the lectin and alternative pathways, allowing for preservation of the important immune surveillance activities.1-3
Learn more about the MOA for ENJAYMOReferences: 1. ENJAYMO. Prescribing information. 2. Shi J, Rose EL, Singh A, et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123(26):4015-4022. doi:10.1182/blood-2014-02-556027 3. Panicker S, Shi J, Rose E, et al. TNT009, a classical complement pathway specific inhibitor, prevents complement dependent hemolysis induced by cold agglutinin disease patient autoantibodies. Blood. 2013;122(21):42. doi:10.1182/blood.V122.21.42.42
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients.1
Reference: 1. ENJAYMO. Prescribing information.